The importance of soil pH in blueberry fields

The content of this article 'The importance of soil pH in blueberry fields' was prepared by the National Sustainable Agriculture Information Service and has been revised and republished by FreshFruitPortal.com.
Blueberries are distinct among fruit crops in their soil and fertility requirements. As members of the Rhododendron family, blueberries require acidic (low pH) soil, preferably in the 4.8 to 5.5 pH range.
When soil pH is appreciably higher than 5.5, iron chlorosis often results; when soil pH drops below 4.8, the possibility of manganese toxicity arises. In either case, plants do not perform well.
Blueberries have a relatively low nitrogen requirement and thrive on organic fertilizers. Soil pH also plays a significant role in nitrogen management for blueberries.
Research shows that blueberries prefer soil and fertilizer nitrogen in the ammonium form, absorbing and using it much more efficiently than nitrate nitrogen—the form preferred by most other commercial crop plants.
Neutral and high-pH soils favor nitrification—the rapid conversion of ammonium nitrogen to nitrate through the activity of nitrifying microorganisms.
In acidic soil, however, the ammonium form of nitrogen predominates and is readily available to blueberries.
For instance, when a slow-release organic fertilizer like fishmeal is applied, the nitrogen in the proteins is converted first into ammonium.
This ammonium—which would rapidly convert to nitrate under neutral soil conditions and be leached out of the root zone—tends to remain in the desired, ammoniated form and thus be held in the soil for uptake.
Perhaps the most common method of lowering soil pH in organic culture is by applying sulfur.
Pre-plant incorporation of sulfur to lower the pH to an optimal blueberry range of 4.8 to 5.5 should be based on a soil pH test.
Because soil pH is subject to considerable seasonal fluctuation—especially on cropped soils—it is advisable to do soil sampling and testing in winter or very early spring, when biological activity is low.
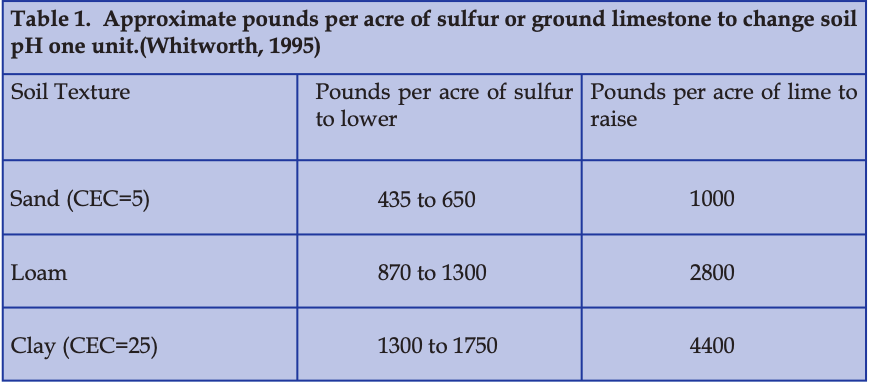
Table 1 provides guidelines for sulfur or lime to raise or lower pH on different types of soil.
Powdered sulfur takes about one year to oxidize and reduce soil pH. Prilled sulfur takes somewhat longer.
Limestone, used to increase pH, requires several months to a year to effect changes in pH, and reactive time is highly dependent on pH, and reactive time is highly dependent on the fineness of the grind.
Single applications of sulfur should not exceed 400 pounds per acre.
Best results are obtained by applying up to 200 pounds in spring, followed by up to 200 in the fall, for as many applications as are required to deliver the total amount. It is advisable to re-test the soil one year after each application to determine whether additional acidification is necessary (Pritts and Hancock, 1992).
Organic growers should be conservative in the application of soil sulfur. Sulfur has both fungicidal and insecticidal actions and can detrimentally affect its biology if overused.
Organic growers sometimes increase their applications of peat moss at planting time, since it too is soil acidified (pH 4.8), reducing the need for sulfur.
The Ozark Organic Growers Association suggests as much as 5 to 10 gallons of peat moss per blueberry plant. While costly, peat is resistant to decomposition and provides the benefit of soil humus.
Those seeking alternatives to sphagnum peat moss might consider pine bark or similar amendments incorporated in the planting rows or holes.
While less desirable than sphagnum peat moss, pine bark often can be obtained locally at a much lower cost.
It is advisable to monitor the pH over time because production practices can cause gradual changes to occur.
Irrigation water often contains calcium and magnesium, which may cause soil pH to creep upwards, while repeated use of acidifying fertilizers, such as cottonseed meal, may lower pH (Spiers and Braswell, 1992).
Fortunately, the presence of abundant organic materials such as peat and the breakdown products of sawdust and woodchip mulches tend to buffer soil pH.
Several organic growers have even observed that blueberries grown in high organic matter soils will perform well at a pH as high as 6.0 with few apparent problems.
As a result, additional sulfur (or lime, for that matter) seldom is needed.
When needed, sulfur is usually applied as a top dressing, but the delivery of soluble sulfur through drip irrigation lines also is an option.
Vinegar or citric acid solutions may also be applied through drip lines to provide acidity.
Source: National Sustainable Agriculture Information Service